One of the oldest methods used for the quantitation of drug molecules is radiometric analysis. This generally involves quantitation of radiation from beta-emitting radioactive isotopes such as 14C, 3H or 32P. Radiometric analysis is one of the most precise, sensitive, and efficient detection methods; however, there are many technical and social challenges with using this technology. Radiometric analysis begins with a radioactive isotope being incorporated into the drug molecule via a chemical synthesis procedure. Generally a specific carbon atom is replaced with 14C, or a specific hydrogen atom is replaced with 3H. The presence (or absence) of the radioactive isotope can be detected which directly correlates with the presence or absence of the drug molecule.
Radiometric analysis is normally performed using liquid scintillation counting (See my previous post on accelerated mass spectrometry for a newer method of radiometric analysis). Scintillation detection is primarily “light” detection, meaning that it detects the number of light particles. Light is produced when the radioactive isotopes emit beta particles that excite fluor molecules that emit light. The light is detected using a photomultiplier tube and converted into “counts” or “disintegrations”. Thus the results are presented as counts per minute (cpm) or disintegrations per minute (dpm).
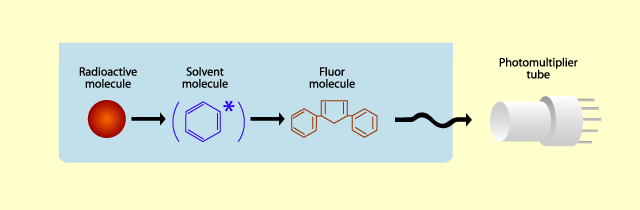
Scintillation Process
Counts refers to the number of light particles counted and disintegrate refers to the number of radioactive isotopes that have disintegrated. The use of light requires that analysis occur in an area of the lab devoid of light. Often scintillation counters are placed in rooms without windows, and they always should be run with the lid closed to prevent ambient light from reaching the photomultiplier tube and affecting the results.
The cpm and dpm results can be interpreted by directly comparing the number of cpm or dpm for a sample relative to the cpm or dpm of a standard with a known amount of radiolabeled drug. In other words, there is a constant dpm/ng of drug, making the determination of the concentration a simple matter of converting dpm to ng, and then dividing by the volume of the original sample to get ng/mL. (Volume refers to the volume of serum or plasma added to the scintillation vial.)
Advantages of radiometric detection include:
- The detection method can be used for multiple matrices (e.g., plasma, serum, blood, tissue, feces, etc.)
- Detection is rapid (sample counting usually takes <5 minutes)
- Detection limits are very low (background is ~20 dpm which is often sub-femtogram levels, depending on labeling efficiency)
- Detection is precise because only the radioisotope of interest is detected
Disadvantages of radiometric detection include:
- Parent and metabolites have same response profile and cannot be distinguished
- Contamination of sample with other radioisotopes can occur
- Synthesis of radiolabeled drug molecules can be expensive and slow
As I mentioned early in the post, there are some social issues with using radiometric analysis. Radioactive isotopes must be handled using specialized procedures, containers and disposal equipment. The waste from experiments with radioisotopes cannot be disposed in standard landfills. In addition, special licenses and training are required to handle radioisotopes. Furthermore, radioisotopes have the potential to cause genetic mutations in animals (including humans), therefore, long-term studies with radiolabeled drugs are not possible, and usually only a single dose of radiolabeled drug is used. All of these factors often lead to avoidance of radioisotopes to reduce the burden on society of handling these materials.
The use of modeling and simulation (M&S) in drug development has evolved from being a research nicety to a regulatory necessity. Today, modeling and simulation is leveraged to some extent, across most development programs to understand and optimize key decisions related to safety, efficacy, dosing, special populations, and others.
Read our white paper to learn about the many benefits of M&S across a drug development program.



