PK is the abbreviation for pharmacokinetics. TK is the abbreviation for toxicokinetics. Some consider these to be distinct specialties while others consider them to be the same. Let me explain and then I would like to see what you think.
Pharmacokinetics generally deals with doses that are in a therapeutic range. Thus common dose ranges will include the no pharmacologic effect level on the low end, and the maximum pharmacologic effect level on the high end.
Toxicokinetics is the study of systemic exposure during toxicological experiments. The objective is to quantify the systemic exposure and potentially relate that to toxicology findings. The comparison of toxicology exposure parameters to therapeutically effective exposure parameters gives rise to the idea of a therapeutic window, or the space between efficacy and toxicity. This is depicted in a theoretical example below.
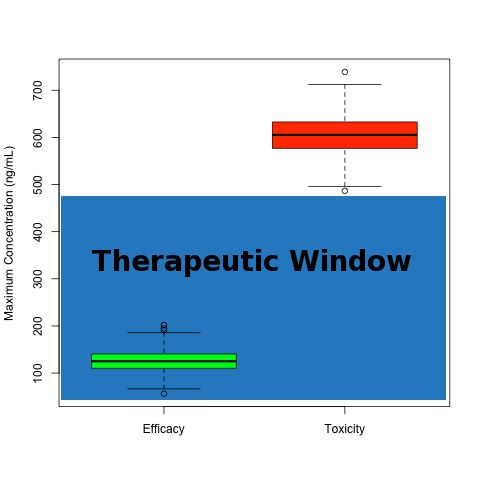
Therapeutic Window
Because of the large doses administered in TK studies, some believe that the TK parameters differ from PK parameters. Toxicology doses saturate all nonlinear processes such as drug absorption, drug transport, drug metabolism and drug elimination. Thus, due to the saturation of physiologic processes, exposure tends to increase proportionally with the dose.
Because of the known saturation of various processes, often only basic TK information is collected. This includes maximum exposure (Cmax), total exposure (AUC), and terminal elimination half-life (t1/2). Other parameters such as volume of distribution and clearance are not calculated in TK studies.
Now that you know the similarities and differences between PK and TK, what do you think? Are these distinct specialties or not? Let me know what you think by commenting on my blog post.
Historically, toxicity testing has been conducted by giving lab animals high doses of chemicals and observing them for adverse events. But quantifying the risks chemicals pose to humans based on animal studies is problematic as the chemical doses are often orders of magnitude higher than environmental levels. Moreover, this process is slow, expensive, and ethically questionable.
Access your free PK/PD resource
Authored by Prof. Johan Gabrielsson, this trusted eBook provides comprehensive insights into pharmacokinetics and pharmacodynamics. Simply register with your professional or academic email to access the standard book “Pharmacokinetic and Pharmacodynamic Data Analysis” – completely free!




