250 Label claims using PBPK without the need for additional clinical studies
“Clinical Studies and Model-Informed Approaches.”
This is the language typically used by the US FDA on drug labels to describe how clinical pharmacology decisions have been reached. Much more detail can be found in the Office of Clinical Pharmacology (OCP) review that supports each label, which is typically posted a few months after each novel drug approval. In these documents, you can review the use of the Simcyp Simulator to support decisions on dosing, safety (notably drug-drug interactions), guidance for use with special populations, and formulations.
Simcyp has now achieved 80+ examples of drugs that have leveraged the Simcyp Simulator for labeling decisions using PBPK for novel drugs. That same Simcyp Simulator has been used on additional indications, line extensions, and complex generic drugs to advance approval and patient access to medicines.
We believe that this list of 80 comprises all novel drugs for which PBPK informed the final drug label.
In other words, Simcyp: 80 vs others at 0.
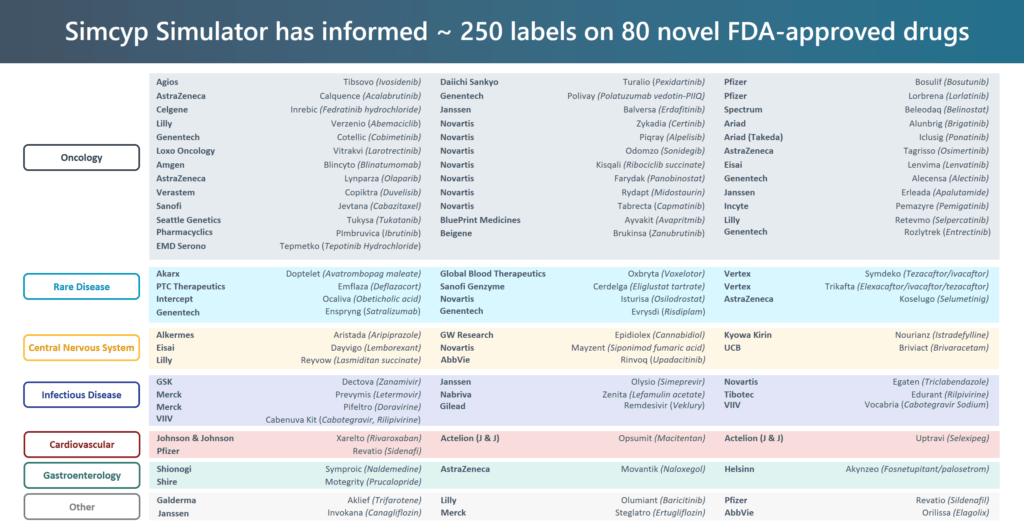
The chart below provides more detail on the type of novel drugs informed by Simcyp. Still mostly small molecules, Simcyp biologics is starting to have a similar impact in that growing field.
- The largest category of oncology represents the outsized focus within the industry. Small patient cohorts, targeted therapies, and breakthrough therapies have propelled PBPK modeling to expedite development;
- Rare disease, much like oncology is a natural for modeling & simulation to maximize the small amount of available clinical data to strengthen the case for meeting unmet medical need;
- CNS and infectious disease are both experiencing a renaissance in development—both with sizeable market opportunities.
For more information we suggest you download Certara’s whitepaper on Simcyp PBPK’s impact on DDI or Simcyp PBPK’s impact on pediatric drug development, or contact us at [email protected].




